Conductivity Test Chemistry . to investigate and explain factors affecting the solubility of different aqueous solutions. Make a table of your results. test for conductivity by carefully placing just the tip of the electrodes in each of the substances in turn. Describe and explain the difference in. in this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. What is conductivity and why should it be measured? to interpret a chemical reaction by observing aqueous solution conductivity. the measurement of solution conductivity is a useful technique for determining the concentrations and mobilities of ions in. Electrical conductivity is based on the flow of.
from www.vernier.com
Electrical conductivity is based on the flow of. test for conductivity by carefully placing just the tip of the electrodes in each of the substances in turn. to investigate and explain factors affecting the solubility of different aqueous solutions. the measurement of solution conductivity is a useful technique for determining the concentrations and mobilities of ions in. Describe and explain the difference in. What is conductivity and why should it be measured? to interpret a chemical reaction by observing aqueous solution conductivity. Make a table of your results. in this class practical, students test the conductivity of covalent and ionic substances in solid and molten states.
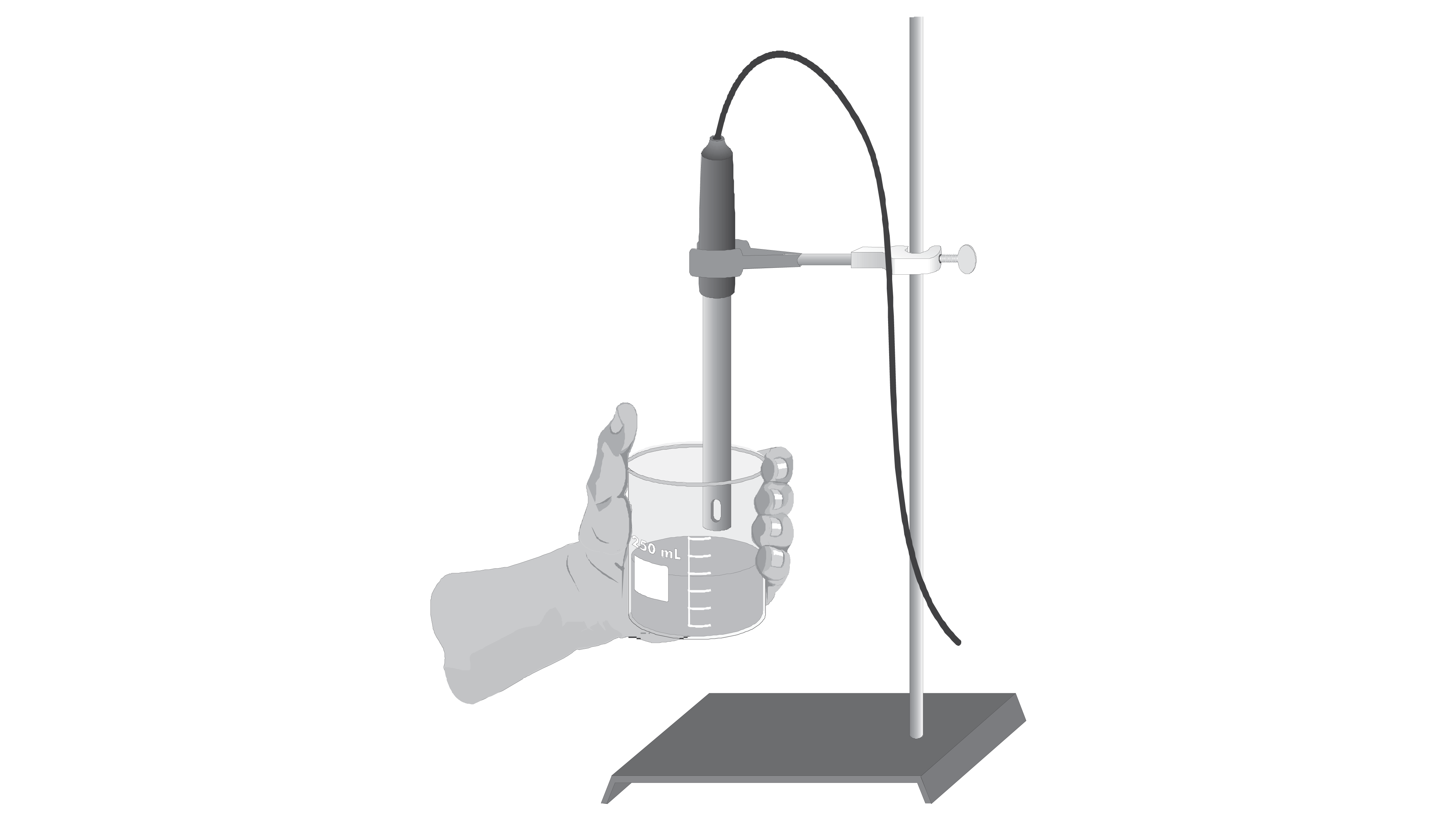
Conductivity of Solutions The Effect of Concentration > Experiment 14
Conductivity Test Chemistry test for conductivity by carefully placing just the tip of the electrodes in each of the substances in turn. Describe and explain the difference in. Electrical conductivity is based on the flow of. in this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. to interpret a chemical reaction by observing aqueous solution conductivity. the measurement of solution conductivity is a useful technique for determining the concentrations and mobilities of ions in. to investigate and explain factors affecting the solubility of different aqueous solutions. test for conductivity by carefully placing just the tip of the electrodes in each of the substances in turn. What is conductivity and why should it be measured? Make a table of your results.
From www.exploratorium.edu
Conductivity Meter Chemistry & Electricity Activity Exploratorium Conductivity Test Chemistry to investigate and explain factors affecting the solubility of different aqueous solutions. Describe and explain the difference in. in this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. test for conductivity by carefully placing just the tip of the electrodes in each of the substances in turn. to. Conductivity Test Chemistry.
From www.youtube.com
Conductivity test for liquid materials YouTube Conductivity Test Chemistry What is conductivity and why should it be measured? test for conductivity by carefully placing just the tip of the electrodes in each of the substances in turn. to interpret a chemical reaction by observing aqueous solution conductivity. in this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. Web. Conductivity Test Chemistry.
From group.chem.iastate.edu
Conductivity Meter Holme Research Group Iowa State University Conductivity Test Chemistry Make a table of your results. the measurement of solution conductivity is a useful technique for determining the concentrations and mobilities of ions in. Electrical conductivity is based on the flow of. to interpret a chemical reaction by observing aqueous solution conductivity. Describe and explain the difference in. test for conductivity by carefully placing just the tip. Conductivity Test Chemistry.
From www.slideserve.com
PPT LIMITED (CLASSICAL) CHEMISTRY METHODS PowerPoint Presentation Conductivity Test Chemistry Describe and explain the difference in. test for conductivity by carefully placing just the tip of the electrodes in each of the substances in turn. to investigate and explain factors affecting the solubility of different aqueous solutions. to interpret a chemical reaction by observing aqueous solution conductivity. the measurement of solution conductivity is a useful technique. Conductivity Test Chemistry.
From www.sciencephoto.com
Conductivity test, 9 of 10 Stock Image C009/4509 Science Photo Conductivity Test Chemistry What is conductivity and why should it be measured? the measurement of solution conductivity is a useful technique for determining the concentrations and mobilities of ions in. Electrical conductivity is based on the flow of. to interpret a chemical reaction by observing aqueous solution conductivity. in this class practical, students test the conductivity of covalent and ionic. Conductivity Test Chemistry.
From www.vernier.com
Conductivity of Solutions The Effect of Concentration > Experiment 14 Conductivity Test Chemistry Electrical conductivity is based on the flow of. test for conductivity by carefully placing just the tip of the electrodes in each of the substances in turn. to investigate and explain factors affecting the solubility of different aqueous solutions. What is conductivity and why should it be measured? the measurement of solution conductivity is a useful technique. Conductivity Test Chemistry.
From www.youtube.com
video chemistry digital conductivity meter with cell atc auto Conductivity Test Chemistry Electrical conductivity is based on the flow of. to interpret a chemical reaction by observing aqueous solution conductivity. to investigate and explain factors affecting the solubility of different aqueous solutions. Make a table of your results. in this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. What is conductivity. Conductivity Test Chemistry.
From www.exploratorium.edu
Conductivity Meter Chemistry & Electricity Activity Exploratorium Conductivity Test Chemistry to interpret a chemical reaction by observing aqueous solution conductivity. test for conductivity by carefully placing just the tip of the electrodes in each of the substances in turn. Make a table of your results. in this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. to investigate and. Conductivity Test Chemistry.
From www.vernier.com
Conductivity of Aqueous Solutions > Experiment 4 from Investigating Conductivity Test Chemistry the measurement of solution conductivity is a useful technique for determining the concentrations and mobilities of ions in. Make a table of your results. Electrical conductivity is based on the flow of. in this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. to investigate and explain factors affecting the. Conductivity Test Chemistry.
From fphoto.photoshelter.com
chemistry conductivity test Fundamental Photographs The Art of Science Conductivity Test Chemistry Electrical conductivity is based on the flow of. to interpret a chemical reaction by observing aqueous solution conductivity. in this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. Make a table of your results. the measurement of solution conductivity is a useful technique for determining the concentrations and mobilities. Conductivity Test Chemistry.
From www.animalia-life.club
Conductivity Chemistry Lab Conductivity Test Chemistry Electrical conductivity is based on the flow of. What is conductivity and why should it be measured? to investigate and explain factors affecting the solubility of different aqueous solutions. the measurement of solution conductivity is a useful technique for determining the concentrations and mobilities of ions in. in this class practical, students test the conductivity of covalent. Conductivity Test Chemistry.
From www.thoughtco.com
Conductivity and Conductive Elements Conductivity Test Chemistry in this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. to interpret a chemical reaction by observing aqueous solution conductivity. the measurement of solution conductivity is a useful technique for determining the concentrations and mobilities of ions in. Describe and explain the difference in. test for conductivity by. Conductivity Test Chemistry.
From fphoto.photoshelter.com
science chemistry conductivity test Fundamental Photographs The Art Conductivity Test Chemistry to interpret a chemical reaction by observing aqueous solution conductivity. Describe and explain the difference in. Make a table of your results. What is conductivity and why should it be measured? Electrical conductivity is based on the flow of. test for conductivity by carefully placing just the tip of the electrodes in each of the substances in turn.. Conductivity Test Chemistry.
From www.sciencephoto.com
Conductivity test Stock Image A250/0128 Science Photo Library Conductivity Test Chemistry to interpret a chemical reaction by observing aqueous solution conductivity. test for conductivity by carefully placing just the tip of the electrodes in each of the substances in turn. in this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. Electrical conductivity is based on the flow of. Make a. Conductivity Test Chemistry.
From fphoto.photoshelter.com
science chemistry conductivity test Fundamental Photographs The Art Conductivity Test Chemistry the measurement of solution conductivity is a useful technique for determining the concentrations and mobilities of ions in. test for conductivity by carefully placing just the tip of the electrodes in each of the substances in turn. in this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. to. Conductivity Test Chemistry.
From sciencephoto.com
Conductivity test Stock Image A250/0129 Science Photo Library Conductivity Test Chemistry to interpret a chemical reaction by observing aqueous solution conductivity. the measurement of solution conductivity is a useful technique for determining the concentrations and mobilities of ions in. What is conductivity and why should it be measured? Electrical conductivity is based on the flow of. in this class practical, students test the conductivity of covalent and ionic. Conductivity Test Chemistry.
From www.scribd.com
Conductivity Test Kit PDF Ph Physical Chemistry Conductivity Test Chemistry in this class practical, students test the conductivity of covalent and ionic substances in solid and molten states. Make a table of your results. to interpret a chemical reaction by observing aqueous solution conductivity. Electrical conductivity is based on the flow of. What is conductivity and why should it be measured? test for conductivity by carefully placing. Conductivity Test Chemistry.
From fphoto.photoshelter.com
science chemistry conductivity test Fundamental Photographs The Art Conductivity Test Chemistry to interpret a chemical reaction by observing aqueous solution conductivity. Electrical conductivity is based on the flow of. test for conductivity by carefully placing just the tip of the electrodes in each of the substances in turn. What is conductivity and why should it be measured? Make a table of your results. to investigate and explain factors. Conductivity Test Chemistry.